Spring Viremia Of Carp
Spring viremia of carp (SVC) is a viral disease that can cause significant mortality in several carp species including the common carp (Cyprinus carpio). These species are raised as a food fish in many countries and koi carp have been selectively bred for the ornamental fish industry. Historically the disease has been a problem in Europe, the Middle East, and Russia, but was reported in koi and feral carp in the United States for the first time in 2002. Listed as a notifiable disease by the World Organization for Animal Health (OIE), diagnosis of SVC in farm raised fish in the US may result in quarantine of the infected fish and depopulation. This information sheet is intended to inform veterinarians, biologists, culturists, and hobbyists about SVC.
What Is Spring Viremia Of Carp?
Spring viremia of carp is an infection caused by Rhabdovirus carpio, a bullet-shaped RNA virus. Natural infections with the virus have been reported in common carp (or koi), (Cyprinus carpio), goldfish (Carassius auratus), grass carp (Ctenopharyngodon idella), bighead carp (Aristichthys nobilis), silver carp (Hypophthalmichthys molitrix), crucian carp (C. carassius), tench (Tinca tinca), sheatfish (Silurus glanis), and rainbow trout (Onchorhynchus mykiss).
Where Is It Found?
Thought to be present in Europe for decades, spring viremia of carp virus (SVCv) was initially diagnosed in Yugoslavia (Fijan et al. 1971). Since then, it has been identified in other European countries, Russia, Brazil, the Middle East, China, and North America.
In the US, SVC has been diagnosed both in farmed koi and feral common carp. In 2002, it was diagnosed in farmed koi in North Carolina and in feral carp in Wisconsin. It is important to note there was no connection between the two cases. It was also diagnosed in farmed koi in Washington and Missouri in 2004 and in feral common carp in the Mississippi River in Minnesota in 2007. The most recent SVC finding was in 2011 in feral carp in Minnesota waters. SVC is not believed to be widespread in the US but rather limited to certain areas in wild susceptible populations. For purposes of disease regulation, USDA considers spring viremia of carp a foreign animal disease in the United States.
What Are The Signs Of SVC?
Clinical signs of infection with SVCv are often non-specific. Affected fish may appear lethargic, exhibit decreased respiration rate and loss of equilibrium. Moribund fish have been reported to lie on their sides, often on the bottom of the tank, and when startled swim up but then return to the bottom. Fish are also reported to congregate where there is slow water flow and near pond banks (Fijan 1999).
Other clinical signs may include darkening of the skin; exophthalmia (pop-eye); coelomic distension (dropsy); pale gills; hemorrhages in the gills, skin, and eye; and a protruding vent with a thick mucoid (white to yellowish) fecal cast (see Figure 1). Internally, edema (fluid buildup in organs and in the body cavity) and inflammation in many organs may be present. Pinpoint hemorrhages are often observed in the wall of the gas bladder and other internal organs. The intestine may be severely inflamed and may contain significant amounts of mucus. The spleen is often enlarged. Concurrent infection with bacteria, particularly Aeromonas (A. salmonicida or A. hydrophila), may confuse the diagnosis because fish with this bacterial disease alone will show similar signs of systemic infection such as ascites and hemorrhages.
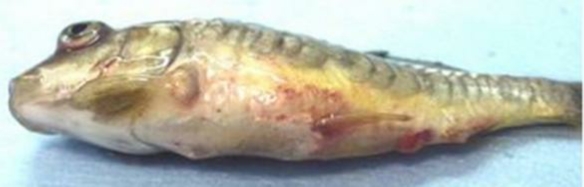
Figure 1: Koi infected with SVCv. Note hemorrhages on body wall.
Transmission Of SVCv:
The rhabdovirus that causes SVC enters the fish through the gills, replicating in gill epithelium and spreading to internal organs (Ahne 1978; Baudouy et al. 1980). The virus is distributed to other susceptible fish via mucoid casts in feces from infected fish. Blood-sucking parasites, including leeches and the fish louse Argulus, have been implicated in spreading the disease (Pfeil-Putzien 1977; Ahne 1985). Mechanical transmission by aquatic animals, birds, and equipment is suspected because of the longevity of the virus in water and mud and following desiccation (Ahne 1982a; Ahne 1982b). The virus can survive freezing for one month at -20°C (-4°F); thus, frozen infected fish that are fed to piscivorous fishes or other animals may spread the virus to other locations (OIE 2015a).
Experimental transmission has been accomplished by co-habitation (housing infected fish with un-infected fish), intracranial (in the head) and intracoelomic (in the body cavity) injection, intubation of the virus into the intestine, and by immersion. However, direct application of the virus to scarified skin has been unsuccessful (Fijan 1972; Fijan et al. 1971; Hill 1977).
The presence of virus in ovarian fluids suggests that vertical transmission (from female parent to offspring) may be possible (Fijan 1999), but there is no evidence this has occurred.
Fish that survive an outbreak of SVCv may develop immunity to the virus. However, they can shed virus and thus serve as a source of virus to unexposed fish. Fish exposed to virus should be considered to be carriers. The length of time carrier fish can shed virus is unknown.
Factors That Influence Disease:
Young fish are more susceptible to infection with SVCv; mortality can reach 70% in yearling carp (Plumb 1999; OIE 2015a). Adult fish can also be affected, but usually to a lesser degree.
Although other factors, such as age, can determine how severely the disease will affect a population, the temperature at which fish become infected, temperature fluctuations during the infective period, and the ability of the fish to mount a timely immune response seem to be the most important components for SVC.
In natural outbreaks, mortalities were confirmed in spring of 1969 and 1970 in Yugoslavia when water temperatures ranged from 12°C to 22°C (54°F to 72°F). The optimum temperature for viral replication in vitro is 20 to 22°C (68 to 72°F); however, this is also an optimum temperature range for immune function of susceptible species (Fijan 1999). Clinical and experimental data indicate that maximum mortality can be expected at water temperatures between 10 to 18°C (50 to 64°F) (Fijan 1999; McAllister 1993).
Fish that are exposed to physiological stressors such as crowding, handling, poor water quality, malnutrition, and sudden temperature changes are more susceptible because of resulting immune system suppression.
How Is SVCv Diagnosed?
Diagnosis of SVCv can be accomplished by several methods. A direct method is to isolate the virus using fathead minnow (FHM) or epithelioma papillosum of carp (EPC) cell lines. If the virus is present, it will cause the cells to degenerate and round (see Figure 2). To confirm that SVCv is in the cell line, one or more of indirect test methods should be performed by a federally approved laboratory. Indirect methods for SVCv diagnosis include enzyme-linked immunosorbent assay (ELISA), virus neutralization, reverse-transcription polymerase chain reaction (rt-PCR), and immunofluorescence of suspect tissue.
Suspect cases of SVC in the US can be sent to any lab that can test for the disease; however, samples collected for the purpose of meeting export requirements must be sent to a USDA-approved lab—https://www.aphis.usda.gov/animal_health/lab_info_services/downloads/ApprovedLabs_Aquaculture.pdf.
If a sample tests positive for the virus, it should be submitted to the National Veterinary Services Laboratory (NVSL) in Ames, Iowa—https://www.aphis.usda.gov/animal_health/lab_info_services/downloads/ContactByDisease.pdf.
A catalog of services and fees and other information regarding NVSL is available here:
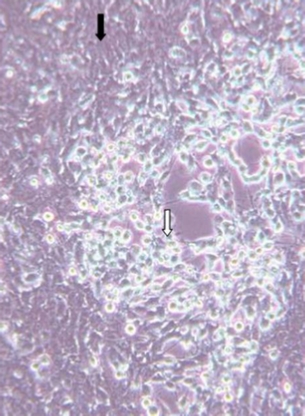
Figure 2.
Photomicrograph of cell culture (epithelioma papillosum of carp) that is infected with SVCv. Black arrow points to cells that appear normal, white arrow to cells dying from SVCv.
How Are SVC Outbreaks Managed?
Antiviral drugs are not available to treat SVC or other viral diseases of cultured fish. Due to the potential severity of the disease and regulatory concerns, depopulation is recommended.
In active outbreaks, efforts are directed at containing and/or quarantining infected and exposed stock, and disinfecting all areas where infected fish were held. However, in some circumstances, this may be difficult. The virus can be infective in mud and water for up to 42 days (Plumb 1999). It can also survive in water without a host for 5 weeks at 10°C (50°F) (OIE 2015a). The fate of the infected and exposed fish will likely be determined by state or federal authorities.
Though the virus is hardy, it can be inactivated by a number of methods. Appendix A lists chemical concentrations and application times to achieve inactivation of the virus. All equipment and tanks, raceways, and ponds should be disinfected. Ponds that have housed infected fish should be fallowed (left un stocked) for a period of time to be determined with regulatory advisory. Re-stocking with non-susceptible species or sentinel, young fish from SVC-free farms is recommended.
The development of genetically resistant strains has been recommended (Fijan 1999), but not pursued (Brown and Bruno 2002). Vaccine development has been attempted in the Czech Republic (Macura et al. 1983) with promising results. One group in the US developed a DNA vaccine for SVCv that was efficacious in laboratory trials (Emmenegger and Kurath 2008). To date, there is no legal vaccine for SVCv in the US.
How Can SVCv Infections Be Prevented?
Bio security measures should be followed especially when working with SVCv-susceptible fish species. These measures should include:
- New fish should be purchased from SVC-free suppliers and farms.
- If surface water is used to supply the farm, it should first be disinfected against SVCv. Well water is considered to be a safe source of water.
- Maintain a dedicated set of equipment for SVC-susceptible species and disinfect it between ponds or tanks.
- Animals such as birds, livestock, pets, etc., should be restricted from access to ponds or tanks.
- Restrict visitors. Only farm personnel should have access to ponds or tanks, and they should be trained in bio security measures.
- Because many farms culture multiple fish species, it is highly recommended that SVCv-susceptible species be kept separate from non-susceptible species.
- Perform routine health assessments including SVCv testing on susceptible populations.
Regulatory Considerations:
Spring viremia of carp is listed as a notifiable disease by the OIE in the International Aquatic Animal Health Code (OIE 2015b). It is also a notifiable disease in the United States. See https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/monitoring-and-surveillance/sa_disease_reporting/ct_disease_list
Prompt notification of the state veterinarian's office and appropriate USDA-APHIS Veterinary Services officials is mandatory by accredited veterinarians and laboratories. It is also on Florida’s list of reportable diseases: http://www.freshfromflorida.com/Divisions-Offices/Animal-Industry/Business-Services/Veterinarians/Reportable-Animal-Diseases.
Appendix A; Methods to inactivate SVCv (Smail and Munro 1989; Fijan 1999; OIE 2015a)
- 3% formalin for 5 minutes
- oxidizing agents
- ozone
- Virkon® Aquatic
- detergents
- sodium dodecyl sulfate
- non-ionic detergents
- sodium hypochlorite (chlorine at 540 ppm for 20 minutes)
- organic iodophors for 30 minutes
- benzalkonium chloride (100 ppm for 20 minutes)
- chlorhexidine gluconate for 20 minutes
- sodium hydroxide for 10 minutes
- gamma and ultraviolet irradiation
- extreme pH (pH 3 for 3 hours or pH 12 for 10 minutes)
- heating at 60°C (140°F) for 30 minutes
